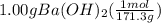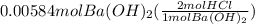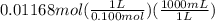Chemistry, 13.07.2019 21:30 ansuaprajita1506
Calculate the volume of 0.100 m hcl required to neutralize 1.00 g of ba(oh)2 (molar mass = 171.3 g/mol).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Calculate the volume of 0.100 m hcl required to neutralize 1.00 g of ba(oh)2 (molar mass = 171.3 g/m...
Questions






Mathematics, 05.09.2019 17:20


English, 05.09.2019 17:20

Mathematics, 05.09.2019 17:20

Mathematics, 05.09.2019 17:20

Mathematics, 05.09.2019 17:20




Mathematics, 05.09.2019 17:30





Physics, 05.09.2019 17:30

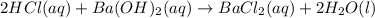
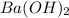 react in 2:1 mol ratio.
react in 2:1 mol ratio.