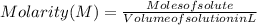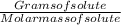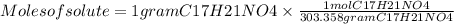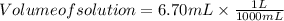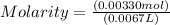Chemistry, 13.07.2019 20:00 myrkaxsanchezz
The free-base form of cocaine has a solubility of 1.00 g in 6.70 ml ethanol (ch3ch2oh). calculate the molarity of a saturated solution of the free-base form of cocaine in ethanol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
You know the right answer?
The free-base form of cocaine has a solubility of 1.00 g in 6.70 ml ethanol (ch3ch2oh). calculate th...
Questions

Social Studies, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30

Social Studies, 17.09.2019 17:30

Biology, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30

Biology, 17.09.2019 17:30


English, 17.09.2019 17:30


Computers and Technology, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30

Computers and Technology, 17.09.2019 17:30



History, 17.09.2019 17:30