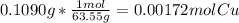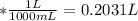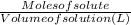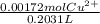Chemistry, 13.07.2019 20:00 masonorourke
A0.1090 g sample of copper metal is dissolved in 60. ml of concentrated hno3 to form cu2+ ions and then water is added to make a total volume of 203.1 ml. (calculate the molarity of cu2+.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1


Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
A0.1090 g sample of copper metal is dissolved in 60. ml of concentrated hno3 to form cu2+ ions and t...
Questions

Mathematics, 13.11.2020 01:00


English, 13.11.2020 01:00

Social Studies, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00

Social Studies, 13.11.2020 01:00

Computers and Technology, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00

History, 13.11.2020 01:00



Mathematics, 13.11.2020 01:00

Arts, 13.11.2020 01:00

Geography, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00

Advanced Placement (AP), 13.11.2020 01:00