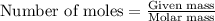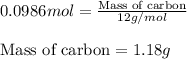Chemistry, 13.07.2019 18:30 naomicervero
C(s)+o2(> co2(g) how many grams of carbon should be burned in an excess of oxygen at stp to obtain 2.21 l of carbon dioxide? 1.18 g 2.21 g 4.12 g 4.34 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
C(s)+o2(> co2(g) how many grams of carbon should be burned in an excess of oxygen at stp to obtai...
Questions

Mathematics, 06.02.2022 04:00


Social Studies, 06.02.2022 04:00

Mathematics, 06.02.2022 04:00

Mathematics, 06.02.2022 04:00

Geography, 06.02.2022 04:00






Mathematics, 06.02.2022 04:00


Mathematics, 06.02.2022 04:00

Business, 06.02.2022 04:00

Biology, 06.02.2022 04:00

Physics, 06.02.2022 04:00

Mathematics, 06.02.2022 04:00

Mathematics, 06.02.2022 04:00

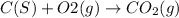
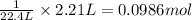 of carbon dioxide gas.
of carbon dioxide gas.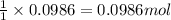 of carbon.
of carbon.