
Chemistry, 13.07.2019 18:30 adyenamaie02
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphorus and excess oxygen. in part b, you found the amount of product (3.40 mol p2o5 ) formed from the given amount of oxygen and excess phosphorus. now, determine how many moles of p2o5 are produced from the given amounts of phosphorus and oxygen. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphor...
Questions


History, 18.03.2021 21:00

Mathematics, 18.03.2021 21:00





Mathematics, 18.03.2021 21:00




Mathematics, 18.03.2021 21:00

Mathematics, 18.03.2021 21:00


Chemistry, 18.03.2021 21:00



Mathematics, 18.03.2021 21:00


English, 18.03.2021 21:00

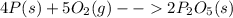
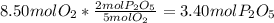
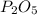 is formed from P (limiting reactant):
is formed from P (limiting reactant):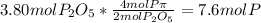
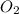 =
= 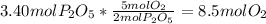
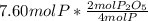
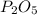 produced from P are
produced from P are 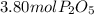
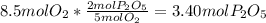
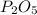 produced will be 3.40 mol
produced will be 3.40 mol

