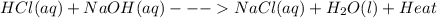Chemistry, 13.07.2019 18:30 preservations
When hcl(aq) and naoh(aq) are mixed in a beaker, the beaker feels warm to the touch. what is known about the enthalpy of this reaction? view available hint(s) when and are mixed in a beaker, the beaker feels warm to the touch. what is known about the enthalpy of this reaction? heat is absorbed from the surroundings. δh is positive. the reaction is exothermic. the reaction is endothermic?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
When hcl(aq) and naoh(aq) are mixed in a beaker, the beaker feels warm to the touch. what is known a...
Questions


Chemistry, 17.11.2020 14:00

Engineering, 17.11.2020 14:00

Health, 17.11.2020 14:00

English, 17.11.2020 14:00

Biology, 17.11.2020 14:00

Chemistry, 17.11.2020 14:00

History, 17.11.2020 14:00


English, 17.11.2020 14:00

English, 17.11.2020 14:00

English, 17.11.2020 14:00


Mathematics, 17.11.2020 14:00

English, 17.11.2020 14:00

English, 17.11.2020 14:00

English, 17.11.2020 14:00

History, 17.11.2020 14:00