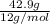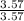Consider 100.0-g samples of two different compounds consisting only of carbon and oxygen. one compound contains 27.2 g of carbon and the other has 42.9 g of carbon. how can these data support the law of multiple proportions if 42.9 is not a multiple of 27.2? show that these data support the law of multiple proportions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 13:30
Asap a 50.0 ml soap bubble is blown in a 27.0°c room. it drifts out an open window and lands in a snow bank at -3.0°c. what is its new volume?
Answers: 1
You know the right answer?
Consider 100.0-g samples of two different compounds consisting only of carbon and oxygen. one compou...
Questions



SAT, 01.12.2021 03:30

Chemistry, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30


Computers and Technology, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30



Chemistry, 01.12.2021 03:30


Mathematics, 01.12.2021 03:30


Biology, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30


Mathematics, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30

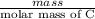

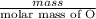
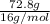

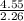
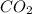 .
.