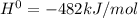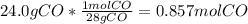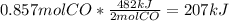Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
The value of δh° for the reaction below is -482 kj. calculate the heat (kj) released to the surround...
Questions



Mathematics, 25.09.2019 19:30

Mathematics, 25.09.2019 19:30

Geography, 25.09.2019 19:30

Chemistry, 25.09.2019 19:30

Geography, 25.09.2019 19:30

Mathematics, 25.09.2019 19:30

Health, 25.09.2019 19:30


Mathematics, 25.09.2019 19:30




Biology, 25.09.2019 19:30

History, 25.09.2019 19:30




Mathematics, 25.09.2019 19:30

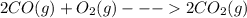 Δ
Δ