Chemistry, 13.07.2019 18:30 sindy35111
Given that the freezing point depression constant for water is 1.86°c kg/mol, calculate the change in freezing point for a 0.907 m sugar solution.

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Given that the freezing point depression constant for water is 1.86°c kg/mol, calculate the change i...
Questions


Computers and Technology, 04.04.2020 11:08

Mathematics, 04.04.2020 11:08


Arts, 04.04.2020 11:08

Biology, 04.04.2020 11:08









Biology, 04.04.2020 11:08


Mathematics, 04.04.2020 11:08



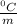
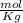 )
)