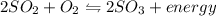Chemistry, 13.07.2019 18:00 Woodsydal2390
Which situation would cause the following equilibrium reaction to decrease the formation of the products? 2so2 (g) + o2 (g) two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2so3 (g) + energy increase the temperature decrease the volume increase the pressure decrease the temperature

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1

Chemistry, 23.06.2019 17:00
What statement about the energy of a phase change is true
Answers: 1
You know the right answer?
Which situation would cause the following equilibrium reaction to decrease the formation of the prod...
Questions

Biology, 18.12.2021 19:40

Advanced Placement (AP), 18.12.2021 19:40

Mathematics, 18.12.2021 19:40





Mathematics, 18.12.2021 19:40






Mathematics, 18.12.2021 19:50

Mathematics, 18.12.2021 19:50

English, 18.12.2021 19:50