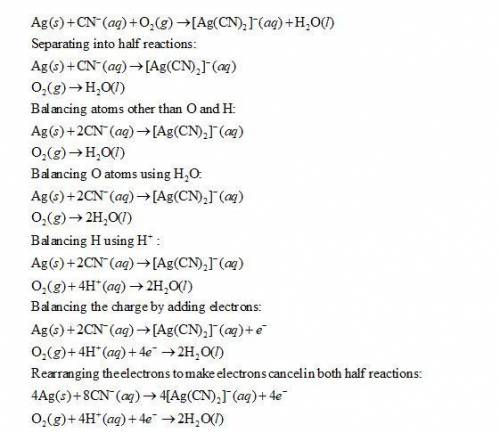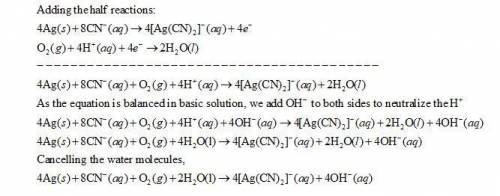Chemistry, 13.07.2019 15:00 brandonm39
Balance the following redox equation, identifying the element oxidized and the element reduced. show all of the work used to solve the problem. ag + cn- + o2 yields ag(cn)2- + h2o

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 09:00
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1

Chemistry, 23.06.2019 10:00
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1

Chemistry, 23.06.2019 15:30
How is the electron sea model of metallic bonding different from the band theory? how are they the same? give at least one similarity and one difference between the models
Answers: 3
You know the right answer?
Balance the following redox equation, identifying the element oxidized and the element reduced. show...
Questions

Spanish, 24.08.2020 06:01


Mathematics, 24.08.2020 06:01

Mathematics, 24.08.2020 06:01


Mathematics, 24.08.2020 06:01


Mathematics, 24.08.2020 06:01

Chemistry, 24.08.2020 06:01

Mathematics, 24.08.2020 06:01


Medicine, 24.08.2020 06:01








![Ag(s) + CN^{-}(aq) + O_{2}(aq) -- [Ag(CN)_{2}]^{-} (aq) + H_{2}O(l)](/tpl/images/0085/1459/07ce2.png)