
Chemistry, 13.07.2019 11:30 heggestade
A100 gram glass container contains 200 grams of water and 50.0 grams of ice all at 0°c. a 200 gram piece of lead at 100°c is added to the water and ice in the container. what is the final temperature of the system? (specific heat of ice = 2,000 j/kg°c , specific heat of water = 4,186 j/kg°c, heat of fusion of water = 333.7 kj/kg, specific heat of glass = 837.2 j/km°c, specific heat of lead = 127.7 j/km°c)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
A100 gram glass container contains 200 grams of water and 50.0 grams of ice all at 0°c. a 200 gram p...
Questions

Medicine, 28.05.2021 18:20

Social Studies, 28.05.2021 18:20

English, 28.05.2021 18:20

Mathematics, 28.05.2021 18:20

Mathematics, 28.05.2021 18:20



Mathematics, 28.05.2021 18:20

World Languages, 28.05.2021 18:20


Business, 28.05.2021 18:20


Computers and Technology, 28.05.2021 18:20

Mathematics, 28.05.2021 18:20

Computers and Technology, 28.05.2021 18:20

SAT, 28.05.2021 18:20


Spanish, 28.05.2021 18:20

Spanish, 28.05.2021 18:20

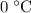
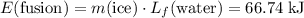 and
and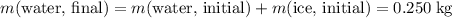
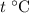 . Thus
. Thus 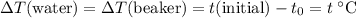
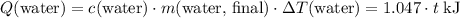 (converted to kilojoules)
(converted to kilojoules) 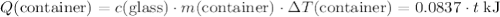
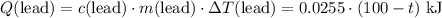
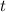 .
. 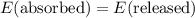
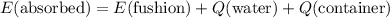
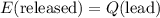
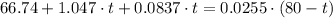
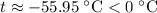 which goes against the initial assumption. Implying that the final temperature does not go above the melting point of water- i.e.,
which goes against the initial assumption. Implying that the final temperature does not go above the melting point of water- i.e., 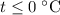 . However, there's no way for the temperature of the system to go below
. However, there's no way for the temperature of the system to go below 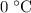 ; doing so would require the removal of heat from the system which isn't possible under the given circumstance; the ice-water mixture experiences an addition of heat as the hot block of lead was added to the system.
; doing so would require the removal of heat from the system which isn't possible under the given circumstance; the ice-water mixture experiences an addition of heat as the hot block of lead was added to the system.


