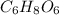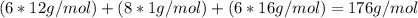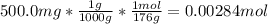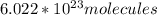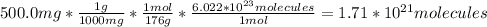Chemistry, 13.07.2019 11:30 samueltaye
Ascorbic acid, of vitamin c (c6h8o6), is an essential vitamin. it cannot be stored by the body and must be present in the diet. what is the molar mass of ascorbic acid? vitamin c tablets are taken as a dietary supplement. if a typical tablet contains 500.0 mg of vitamin c, how many moles and how many molecules of vitamin c does it contain?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Ascorbic acid, of vitamin c (c6h8o6), is an essential vitamin. it cannot be stored by the body and m...
Questions

Computers and Technology, 01.03.2021 04:20



Biology, 01.03.2021 04:20

History, 01.03.2021 04:20

Geography, 01.03.2021 04:20


Social Studies, 01.03.2021 04:20

Biology, 01.03.2021 04:20


Mathematics, 01.03.2021 04:20





Mathematics, 01.03.2021 04:20

Mathematics, 01.03.2021 04:20

Mathematics, 01.03.2021 04:20

Social Studies, 01.03.2021 04:20

Arts, 01.03.2021 04:20