Chemistry, 13.07.2019 09:30 paolaz80045
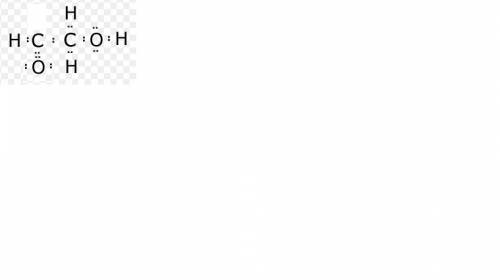
Draw it draw lewis dot structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. explain what makes the other molecules nonsensical, considering the num- ber of bonds each type of atom can make

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Draw it draw lewis dot structures for each hypothetical molecule shown below, using the correct numb...
Questions




English, 27.06.2020 23:01






History, 27.06.2020 23:01

Biology, 27.06.2020 23:01

Mathematics, 27.06.2020 23:01



Mathematics, 27.06.2020 23:01


Mathematics, 27.06.2020 23:01


English, 27.06.2020 23:01

Computers and Technology, 27.06.2020 23:01