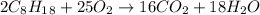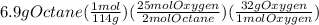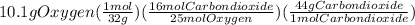Chemistry, 13.07.2019 09:30 cyaransteenberg
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 6.9 g of octane is mixed with 10.1 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon di...
Questions


Mathematics, 28.05.2021 22:30

Geography, 28.05.2021 22:30






English, 28.05.2021 22:30

Mathematics, 28.05.2021 22:30

Mathematics, 28.05.2021 22:30

Mathematics, 28.05.2021 22:30

Mathematics, 28.05.2021 22:30

Mathematics, 28.05.2021 22:30



Medicine, 28.05.2021 22:30

History, 28.05.2021 22:30

Social Studies, 28.05.2021 22:30

Advanced Placement (AP), 28.05.2021 22:30