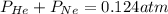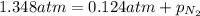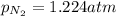Chemistry, 13.07.2019 09:30 shanicejordan
Amixture of he, ne, and n2 gases are a pressure of 1.348. if the pressures of he and ne are 0.124 atm, what is the partial pressure of n2 in the mixture ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
Amixture of he, ne, and n2 gases are a pressure of 1.348. if the pressures of he and ne are 0.124 at...
Questions


Mathematics, 13.11.2019 21:31




Biology, 13.11.2019 21:31



Mathematics, 13.11.2019 21:31


English, 13.11.2019 21:31

Geography, 13.11.2019 21:31



Mathematics, 13.11.2019 21:31

English, 13.11.2019 21:31

English, 13.11.2019 21:31



English, 13.11.2019 22:31

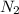 in the mixture is, 1.224 atm
in the mixture is, 1.224 atm
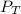 = total partial pressure of
= total partial pressure of 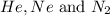 = 1.348 atm
= 1.348 atm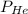 = partial pressure of helium
= partial pressure of helium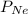 = partial pressure of neon
= partial pressure of neon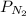 = partial pressure of nitrogen
= partial pressure of nitrogen