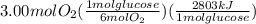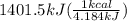Chemistry, 13.07.2019 08:00 zekrader18
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process releases 2803 kj per mole of glucose. when 3.00 mol of oxygen react in this way with glucose, what is the energy release in kcal? (hint: write a balanced equation for the combustion process.)

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1


Chemistry, 23.06.2019 13:40
Match these items with their examples. 1. liquid solution milk 2. solid solution aluminum foil 3. compound soda 4. colloid steel 5. element salt
Answers: 1

Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1
You know the right answer?
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process...
Questions


English, 03.11.2021 07:00


History, 03.11.2021 07:00

Social Studies, 03.11.2021 07:00

Mathematics, 03.11.2021 07:10

Social Studies, 03.11.2021 07:10

Chemistry, 03.11.2021 07:10

History, 03.11.2021 07:10

Mathematics, 03.11.2021 07:10

Biology, 03.11.2021 07:10


Mathematics, 03.11.2021 07:10


Mathematics, 03.11.2021 07:20

Physics, 03.11.2021 07:20


Chemistry, 03.11.2021 07:20