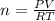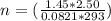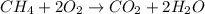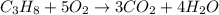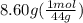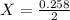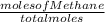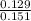A2.50-l flask contains a mixture of methane (ch4) and propane (c3h8) at a pressure of 1.45 atm and 20°c. when this gas mixture is then burned in excess oxygen, 8.60 g of carbon dioxide is formed. (the other product is water.) what is the mole fraction of methane in the original gas mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
A2.50-l flask contains a mixture of methane (ch4) and propane (c3h8) at a pressure of 1.45 atm and 2...
Questions

Mathematics, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

Computers and Technology, 22.04.2021 14:00


Biology, 22.04.2021 14:00




Mathematics, 22.04.2021 14:00

History, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00






Mathematics, 22.04.2021 14:00