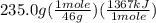Chemistry, 13.07.2019 01:00 marvinc5603
The combustion of one mole of liquid ethanol, ch3ch2oh, produces 1367 kj of heat. calculate how much heat is produced when 235.0 g of ethanol are combusted.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
The combustion of one mole of liquid ethanol, ch3ch2oh, produces 1367 kj of heat. calculate how much...
Questions

Mathematics, 23.08.2019 04:00



Physics, 23.08.2019 04:00

German, 23.08.2019 04:00

Mathematics, 23.08.2019 04:00

Mathematics, 23.08.2019 04:00


History, 23.08.2019 04:00


History, 23.08.2019 04:00

English, 23.08.2019 04:00


Mathematics, 23.08.2019 04:00

Mathematics, 23.08.2019 04:00

Mathematics, 23.08.2019 04:00