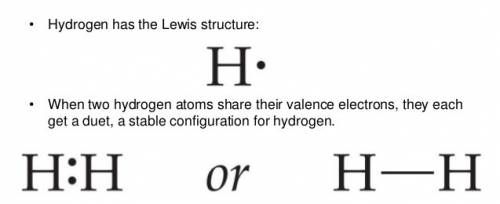
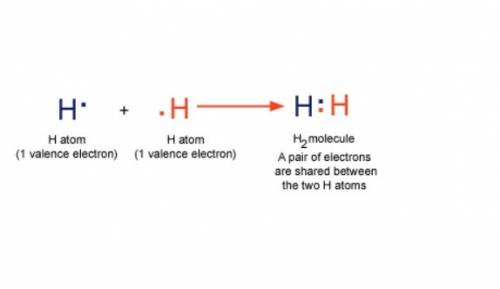
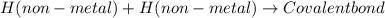Chemistry, 13.07.2019 00:30 monkeys450
What type of bond is joining the two hydrogen atoms? there are 2 hydrogen atoms with a pair of electrons shared between these 2 atoms. what type of bond is joining the two hydrogen atoms? there are 2 hydrogen atoms with a pair of electrons shared between these 2 atoms. covalent hydrophobic hydrophilic hydrogen ionic?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
What type of bond is joining the two hydrogen atoms? there are 2 hydrogen atoms with a pair of elec...
Questions

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Biology, 10.12.2020 01:00

History, 10.12.2020 01:00



Chemistry, 10.12.2020 01:00


Health, 10.12.2020 01:00

English, 10.12.2020 01:00

Chemistry, 10.12.2020 01:00


Advanced Placement (AP), 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Biology, 10.12.2020 01:00