

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
"the osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temp...
Questions

Mathematics, 24.10.2019 02:00




English, 24.10.2019 02:00


Social Studies, 24.10.2019 02:00



History, 24.10.2019 02:00

Geography, 24.10.2019 02:00

Mathematics, 24.10.2019 02:00


Mathematics, 24.10.2019 02:00






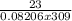 mol/ L
mol/ L

