

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Cadmium metal reacts vigorously with yellow crystals of sulfur to produce a yellow powder of cadmium...
Questions

Mathematics, 26.08.2019 06:30

Biology, 26.08.2019 06:30






Biology, 26.08.2019 06:30

Mathematics, 26.08.2019 06:30

History, 26.08.2019 06:30

History, 26.08.2019 06:30

Mathematics, 26.08.2019 06:30

Mathematics, 26.08.2019 06:30


Chemistry, 26.08.2019 06:30

Mathematics, 26.08.2019 06:30


Biology, 26.08.2019 06:30

Mathematics, 26.08.2019 06:30

Mathematics, 26.08.2019 06:30

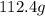 of cadmium with
of cadmium with 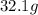 of sulfur =
of sulfur = 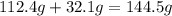
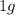 of cadmium forms
of cadmium forms 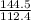 cadmium sulfide.
cadmium sulfide.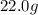 of cadmium will give
of cadmium will give 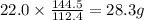 of cadmium sulfide.
of cadmium sulfide.

