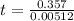Chemistry, 12.07.2019 18:00 JammedBanjo58
Consider the below chemical reaction that occurs via first-order kinetics with a rate constant of 5.12 x 10–3 s–1 at a particular temperature. how long will it take for 30% of substrate a to be consumed? a → b 83.7 s

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2
You know the right answer?
Consider the below chemical reaction that occurs via first-order kinetics with a rate constant of 5....
Questions


Social Studies, 16.10.2019 21:00





Mathematics, 16.10.2019 21:00


Spanish, 16.10.2019 21:00

History, 16.10.2019 21:00

Social Studies, 16.10.2019 21:00

Mathematics, 16.10.2019 21:00

Biology, 16.10.2019 21:00

Mathematics, 16.10.2019 21:00






![ln[A]=-kt+ln[A_0]](/tpl/images/0081/8081/fcf7e.png)
![[A_0]](/tpl/images/0081/8081/9a686.png) is the initial concentration and [A] is final concentration.
is the initial concentration and [A] is final concentration.
![ln[70]=-5.12*10^-^3(t)+ln[100]](/tpl/images/0081/8081/d14d5.png)