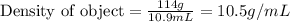In an old trunk, you find a piece of metal that you think may be aluminum, silver, or lead. you take it to a lab, where you find it has a mass of 114 g and a volume of 10.9 cm3. what is the metal you found? assume that densities of aluminium, silver, and lead are 2.70, 10.5, and 11.3 g/ml, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
In an old trunk, you find a piece of metal that you think may be aluminum, silver, or lead. you take...
Questions


Social Studies, 20.01.2021 18:40



English, 20.01.2021 18:40



English, 20.01.2021 18:40



French, 20.01.2021 18:40

English, 20.01.2021 18:40

Social Studies, 20.01.2021 18:40


Physics, 20.01.2021 18:40




Mathematics, 20.01.2021 18:50

Physics, 20.01.2021 18:50

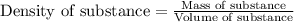
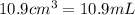 (Conversion factor:
(Conversion factor: 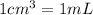 )
)