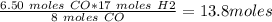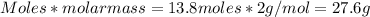Chemistry, 12.07.2019 13:30 nengliangli523
Given: 8co + 17h2 → c8h18 + 8h2o in this chemical reaction, how many grams of h2 will react completely with 6.50 moles of co? express your answer to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
You know the right answer?
Given: 8co + 17h2 → c8h18 + 8h2o in this chemical reaction, how many grams of h2 will react complet...
Questions

Mathematics, 04.06.2020 21:59

Mathematics, 04.06.2020 21:59

Physics, 04.06.2020 21:59





Medicine, 04.06.2020 21:59

History, 04.06.2020 21:59



Mathematics, 04.06.2020 21:59

Mathematics, 04.06.2020 21:59


English, 04.06.2020 21:59

History, 04.06.2020 21:59

English, 04.06.2020 21:59

Mathematics, 04.06.2020 21:59

Mathematics, 04.06.2020 21:59

Mathematics, 04.06.2020 21:59