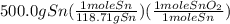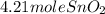Chemistry, 12.07.2019 13:30 juliannxkim
Sno2 + 2h2 → sn + 2h2o tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn? a. 1.57 b. 4.21 c. 634.8 d. 59,350

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1


Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Sno2 + 2h2 → sn + 2h2o tin oxide reacts with hydrogen to produce tin and water. how many moles of sn...
Questions

Chemistry, 06.11.2019 05:31








Mathematics, 06.11.2019 05:31


English, 06.11.2019 05:31

English, 06.11.2019 05:31


Biology, 06.11.2019 05:31

English, 06.11.2019 05:31

Mathematics, 06.11.2019 05:31


English, 06.11.2019 05:31

Social Studies, 06.11.2019 05:31

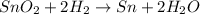
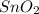 and Sn.
and Sn.