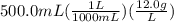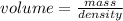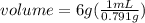Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

You know the right answer?
How many milliliters of pure liquid methanol (ch3oh, mw = 32.04 g/mol) are needed to prepare 500.0 m...
Questions


Mathematics, 12.08.2020 08:01

Biology, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01







Biology, 12.08.2020 08:01









Computers and Technology, 12.08.2020 08:01

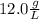 . This has to be made from a given pure liquid methanol with density
. This has to be made from a given pure liquid methanol with density 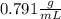 . It asks to calculate the volume of pure liquid methanol.
. It asks to calculate the volume of pure liquid methanol.