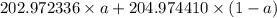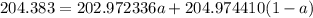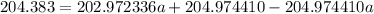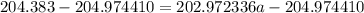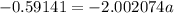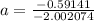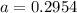Chemistry, 12.07.2019 12:00 tsedeneyaalemu2924
The atomic masses of 203tl and 205tl are 202.972336 and 204.974410 amu, respectively. the average atomic mass of thallium is 204.383 amu. calculate the natural abundances of these two isotopes.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

You know the right answer?
The atomic masses of 203tl and 205tl are 202.972336 and 204.974410 amu, respectively. the average at...
Questions




History, 08.11.2019 04:31

History, 08.11.2019 04:31







English, 08.11.2019 04:31




Biology, 08.11.2019 04:31


History, 08.11.2019 04:31


Social Studies, 08.11.2019 04:31