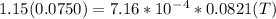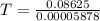Chemistry, 12.07.2019 11:30 ajayfurlow
A75.0-milliliter lightbulb is filled with neon. there are 7.16 × 10-4 moles of gas in it, and the absolute pressure is 116.8 kilopascals after the bulb has been on for an hour. how hot did the bulb get? the temperature of the lightbulb was k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2

You know the right answer?
A75.0-milliliter lightbulb is filled with neon. there are 7.16 × 10-4 moles of gas in it, and the ab...
Questions



Social Studies, 18.11.2020 18:50


Social Studies, 18.11.2020 18:50

History, 18.11.2020 18:50


Mathematics, 18.11.2020 18:50

Chemistry, 18.11.2020 18:50


Mathematics, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50



English, 18.11.2020 18:50


Biology, 18.11.2020 18:50


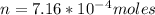
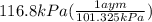
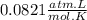 .
.