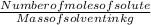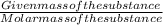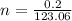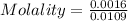Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2


Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
If 0.2 g of nitrobenzene are added to 10.9 g of naphthalene, calculate the molality of the solution....
Questions



Mathematics, 22.01.2020 01:31




English, 22.01.2020 01:31

Mathematics, 22.01.2020 01:31


Mathematics, 22.01.2020 01:31


Biology, 22.01.2020 01:31



English, 22.01.2020 01:31

Mathematics, 22.01.2020 01:31

Mathematics, 22.01.2020 01:31

Social Studies, 22.01.2020 01:31

Spanish, 22.01.2020 01:31

History, 22.01.2020 01:31