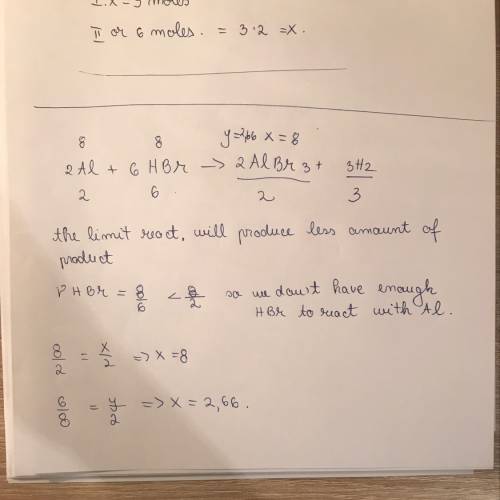Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
50pts consider the reaction 2al + 6hbr → 2albr3 + 3h2. if 8 moles of al react with 8 moles of hbr, w...
Questions

Mathematics, 08.10.2021 21:00





Mathematics, 08.10.2021 21:00

Mathematics, 08.10.2021 21:00







Mathematics, 08.10.2021 21:00



Mathematics, 08.10.2021 21:00

Social Studies, 08.10.2021 21:00

Mathematics, 08.10.2021 21:00

English, 08.10.2021 21:00