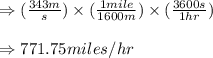Chemistry, 12.07.2019 06:30 cheervolley
The speed of the sound in air at room temperature is 343 m/s. calculate this speed in miles per hour.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
The speed of the sound in air at room temperature is 343 m/s. calculate this speed in miles per hour...
Questions


Mathematics, 09.09.2021 21:20


Biology, 09.09.2021 21:20


Biology, 09.09.2021 21:20

Mathematics, 09.09.2021 21:20

English, 09.09.2021 21:20

Mathematics, 09.09.2021 21:20




History, 09.09.2021 21:20




Mathematics, 09.09.2021 21:20

Mathematics, 09.09.2021 21:20

Mathematics, 09.09.2021 21:20

Mathematics, 09.09.2021 21:20