
Chemistry, 12.07.2019 05:30 cherylmorton7302
Calculate the solubility of benzene in water at 25 c in ppm. the required henry's law constant is 5.6 bar/mol/kg and benzene's saturated vapor pressure is 0.13 bar.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Calculate the solubility of benzene in water at 25 c in ppm. the required henry's law constant is 5....
Questions

SAT, 16.12.2020 01:40

Advanced Placement (AP), 16.12.2020 01:40


English, 16.12.2020 01:40


Mathematics, 16.12.2020 01:40









Biology, 16.12.2020 01:40



Medicine, 16.12.2020 01:40


Mathematics, 16.12.2020 01:40

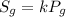
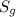 is the solubility of the gas.
is the solubility of the gas.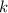 is proportionality constant i.e. Henry's constant.
is proportionality constant i.e. Henry's constant.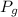 is pressure of the gas.
is pressure of the gas.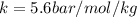 (given)
(given)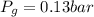 (given)
(given)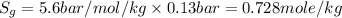
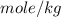 to
to 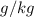 :
: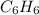 =
= 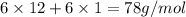
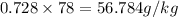
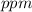 :
: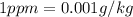
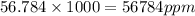 .
.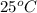 in
in 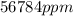 .
.

