
Chemistry, 12.07.2019 05:30 christianconklin22
The gas no reacts with h2, forming n2 and h2o: $$2no(g)+2h2(g) 2h2o(g)+n2(g) if δ[no]/ δt = –24.0 m/s under a given set of conditions, what are the rates of change of [n2] and [h2o]?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2



Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
The gas no reacts with h2, forming n2 and h2o: $$2no(g)+2h2(g) 2h2o(g)+n2(g) if δ[no]/ δt = –24.0 m...
Questions

Mathematics, 27.08.2019 23:00

Mathematics, 27.08.2019 23:00

Mathematics, 27.08.2019 23:00



Mathematics, 27.08.2019 23:00

Social Studies, 27.08.2019 23:00


Biology, 27.08.2019 23:00



Mathematics, 27.08.2019 23:00


Mathematics, 27.08.2019 23:00


History, 27.08.2019 23:00


Mathematics, 27.08.2019 23:00


Social Studies, 27.08.2019 23:00

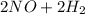 →
→ 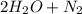 . For this reaction, rate of reaction is decrease in the concentration of NO with respect to time or decrease in the concentration of H₂ with respect to time or increase in the concentration of H₂O with respect to time or increase in the concentration of N₂ with respect to time.
. For this reaction, rate of reaction is decrease in the concentration of NO with respect to time or decrease in the concentration of H₂ with respect to time or increase in the concentration of H₂O with respect to time or increase in the concentration of N₂ with respect to time.![-\frac{1}{2} \frac{d[NO]}{dt}= -\frac{1}{2} \frac{d[H_{2} ]}{dt}=+\frac{1}{2} \frac{d[H_{2} O]}{dt}= +\frac{d[N_{2} ]}{dt}](/tpl/images/0079/8245/dff0b.png)
![\frac{d[NO]}{dt}=24.0 m/s](/tpl/images/0079/8245/5228b.png) . So, rates of change of [N₂]=
. So, rates of change of [N₂]= ![-\frac{1}{2} \frac{d[NO]}{dt}](/tpl/images/0079/8245/831c0.png) =
=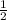 ×24.0 m/s=12 m/s. Rate of change of [H₂O] =
×24.0 m/s=12 m/s. Rate of change of [H₂O] =![+\frac{1}{2} \frac{d[H_{2} O]}{dt}=-\frac{1}{2} \frac{d[NO]}{dt}](/tpl/images/0079/8245/30264.png) = 24.0 m/s.
= 24.0 m/s.

