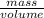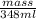Chemistry, 12.07.2019 05:00 mayavue99251
Aluminum has a density of 2.70 g/ml. calculate the mass (in grams) of a piece of aluminum having a volume of 348 ml .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1


Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3
You know the right answer?
Aluminum has a density of 2.70 g/ml. calculate the mass (in grams) of a piece of aluminum having a v...
Questions

Mathematics, 08.04.2021 15:50


History, 08.04.2021 15:50

Mathematics, 08.04.2021 15:50


Mathematics, 08.04.2021 15:50

Geography, 08.04.2021 15:50


History, 08.04.2021 15:50


History, 08.04.2021 15:50



English, 08.04.2021 15:50



SAT, 08.04.2021 15:50

Geography, 08.04.2021 15:50

Mathematics, 08.04.2021 15:50

English, 08.04.2021 15:50