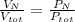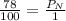Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25.0 ∘c, the n2 component will dissolve in water with a solubility of 4.88×10−4 m. what is the value of henry's law constant for n2 under these conditions? express your answer with the appropriate units. enter the unit m using the compound form mol/l.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25....
Questions



Mathematics, 04.11.2020 21:20

English, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20




Computers and Technology, 04.11.2020 21:20



Mathematics, 04.11.2020 21:20

History, 04.11.2020 21:20

Health, 04.11.2020 21:20

History, 04.11.2020 21:20


Mathematics, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20