
Chemistry, 12.07.2019 02:30 dolahghazali76
A20.7167 g sample of impure magnesium carbonate was heated to complete decomposition according to the equation mgco3(s) → mgo(s) + co2(g). after the reaction was complete, the solid residue (consisting of mgo and the original impurities) had a mass of 16.8817 g. assuming that only the magnesium carbonate had decomposed, how much magnesium carbonate was present in the original sample? answer in units of g. 013 5.0 points the reaction of 8.9 grams of fluo

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
A20.7167 g sample of impure magnesium carbonate was heated to complete decomposition according to th...
Questions


History, 23.06.2019 04:31

Physics, 23.06.2019 04:31

Mathematics, 23.06.2019 04:31


English, 23.06.2019 04:31

Social Studies, 23.06.2019 04:31

Mathematics, 23.06.2019 04:31


Biology, 23.06.2019 04:31



Mathematics, 23.06.2019 04:31

Social Studies, 23.06.2019 04:31


Advanced Placement (AP), 23.06.2019 04:31

History, 23.06.2019 04:31

English, 23.06.2019 04:31

English, 23.06.2019 04:31

Mathematics, 23.06.2019 04:31

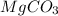 is 20.7167 g. The decomposition reaction is as follows:
is 20.7167 g. The decomposition reaction is as follows:





 ...... (1)
...... (1) and mass of impure
and mass of impure  ...... (2)
...... (2) ...... (3)
...... (3)
 in equation (1),
in equation (1),






