
Acompound contains only carbon, hydrogen, and oxygen. combustion of 9.612 mg of the compound yields 14.41 mg co2 and 3.93 mg h2o. the molar mass of the compound is 176.1 g/mol. what are the empirical and molecular formulas of the compound? (type your answer using the format co2 for co2.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
Acompound contains only carbon, hydrogen, and oxygen. combustion of 9.612 mg of the compound yields...
Questions

English, 20.01.2021 19:30


English, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30



Mathematics, 20.01.2021 19:30

English, 20.01.2021 19:30

English, 20.01.2021 19:30

History, 20.01.2021 19:30

Biology, 20.01.2021 19:30

English, 20.01.2021 19:30



Geography, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30


Mathematics, 20.01.2021 19:30

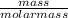 -(1)
-(1)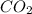 = 14.41 mg (given)
= 14.41 mg (given)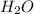 = 3.93 mg (given)
= 3.93 mg (given)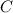 from
from 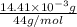
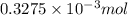
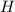 from
from 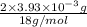
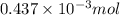
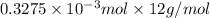
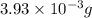
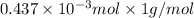
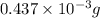
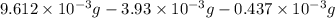
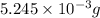
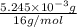
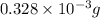
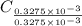
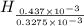
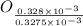
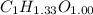
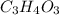
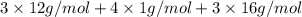
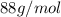
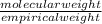 =
= 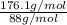
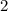
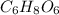 .
.

