
Chemistry, 12.07.2019 01:30 nefertiri64
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indicate about the reaction? a. because a and b are equal, the reaction has reached equilibrium. b. there is more a and b than ab at equilibrium, and the reaction favors the reactants. c. there is more ab than a and b at equilibrium, and the reaction favors the products. d. there is more ab than a and b at equilibrium, and the reaction favors the reactants. e. the reaction is not at equilibrium.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indi...
Questions

Mathematics, 19.10.2019 07:30

History, 19.10.2019 07:30

Social Studies, 19.10.2019 07:30


Computers and Technology, 19.10.2019 07:30

History, 19.10.2019 07:30

English, 19.10.2019 07:30

Mathematics, 19.10.2019 07:30




Biology, 19.10.2019 07:30



Business, 19.10.2019 07:30

Mathematics, 19.10.2019 07:30

History, 19.10.2019 07:30



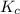 is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
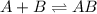
![K_c=\frac{[AB]}{[A][B]}](/tpl/images/0079/1606/5faa1.png)
![K_c=\frac{[0.72]}{[0.14][0.14]}=36.7](/tpl/images/0079/1606/2d664.png)
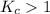 ; then the reaction is product favored.
; then the reaction is product favored.
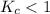 ; then the reaction is reactant favored.
; then the reaction is reactant favored.
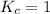 ; then the reaction is in equilibrium.
; then the reaction is in equilibrium.


