
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced equation: (ii)what would be observed if the solution was titrated well past the equivalence point using bromthymolblue as the indicator? (bromthymol blue is yellow in acidic solution and blue in basic solution.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1


You know the right answer?
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced...
Questions

Mathematics, 28.01.2021 22:40


History, 28.01.2021 22:40


Physics, 28.01.2021 22:40

Mathematics, 28.01.2021 22:40

Mathematics, 28.01.2021 22:40



Chemistry, 28.01.2021 22:40



Mathematics, 28.01.2021 22:40

Mathematics, 28.01.2021 22:40


Mathematics, 28.01.2021 22:40



Chemistry, 28.01.2021 22:40

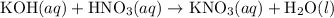
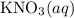 (and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result,
(and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result, 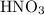 is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and
is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and 

