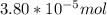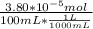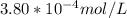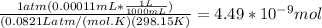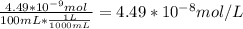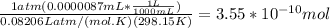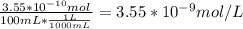A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in find how many moles of he are contained in 5.24 μl at 25.00°c (298.15 k) and 1.000 atm. this number is the molarity of he in the air. (2.11x10-7m) (b) find the molar concentrations of ar, kr, and xe in air at 25°c and 1 atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in f...
Questions



Physics, 10.05.2021 01:30



Mathematics, 10.05.2021 01:30

English, 10.05.2021 01:30

History, 10.05.2021 01:30

Social Studies, 10.05.2021 01:40

Physics, 10.05.2021 01:40






Mathematics, 10.05.2021 01:40




Mathematics, 10.05.2021 01:40

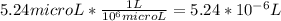
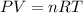
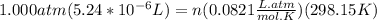
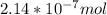
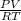
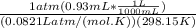 =
=