
Chemistry, 11.07.2019 23:30 steffweikeloyrt00
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of coffee with a temperature of 88.3 degrees c. assume the specific heats of coffee and milk are the same as for water (c = 4.19 j/g•c), and disregard the heat capacity of the container.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of co...
Questions

Chemistry, 12.11.2020 21:30

Computers and Technology, 12.11.2020 21:30



Computers and Technology, 12.11.2020 21:30


Mathematics, 12.11.2020 21:30


Mathematics, 12.11.2020 21:30


Biology, 12.11.2020 21:30

English, 12.11.2020 21:30


English, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30


Mathematics, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30


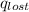 =
=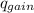 ,
, 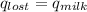 +
+ 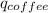
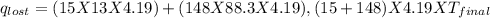 =(15X13X4.19)+(148X88.3X4.19)
=(15X13X4.19)+(148X88.3X4.19)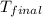 = 81.37 ° C
= 81.37 ° C

