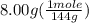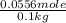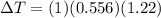Chemistry, 11.07.2019 23:00 kylucienne
The boiling point of pure ethanol, c2h5oh, is 78.4 ∘c. its boiling point elevation constant is 1.22 °c/m. what is the boiling point of a solution formed by dissolving 8.00 g of alpha-naphthol (c10h7oh) in 100.0 g ethanol. 91.3 °c 79.1°c 97.6 °c 78.5°c

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
The boiling point of pure ethanol, c2h5oh, is 78.4 ∘c. its boiling point elevation constant is 1.22...
Questions

Computers and Technology, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01



Arts, 12.08.2020 05:01

History, 12.08.2020 05:01

English, 12.08.2020 05:01

Physics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01


History, 12.08.2020 05:01


Health, 12.08.2020 05:01

Biology, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

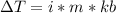
 is the elevation in boiling point
is the elevation in boiling point