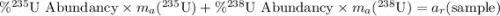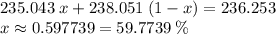Chemistry, 11.07.2019 22:30 gwendallinesikes
Only 235u can be used as fuel in a nuclear reactor, so uranium for use in the nuclear industry must be enriched in this isotope. if a sample of enriched uranium has an average atomic mass of 236.253 amu, what percentage of 235u is present?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 1
You know the right answer?
Only 235u can be used as fuel in a nuclear reactor, so uranium for use in the nuclear industry must...
Questions


Biology, 12.07.2019 13:40

Business, 12.07.2019 13:40






History, 12.07.2019 13:40

Social Studies, 12.07.2019 13:40

Mathematics, 12.07.2019 13:40

History, 12.07.2019 13:40



Biology, 12.07.2019 13:40

Biology, 12.07.2019 13:40

Chemistry, 12.07.2019 13:40

History, 12.07.2019 13:40

Social Studies, 12.07.2019 13:40

Biology, 12.07.2019 13:40

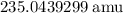 andUranium 238 with a relative atomic mass of
andUranium 238 with a relative atomic mass of 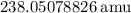
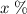 of the uranium atoms in this sample are atoms of
of the uranium atoms in this sample are atoms of 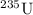 ; given the fact that other isotopes of uranium contribute to a negligible share of mass of the sample (less than
; given the fact that other isotopes of uranium contribute to a negligible share of mass of the sample (less than 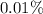 in naturally-occurring samples,)
in naturally-occurring samples,) 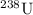 would have contributed to
would have contributed to 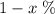 of all atoms in this sample.
of all atoms in this sample.