Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
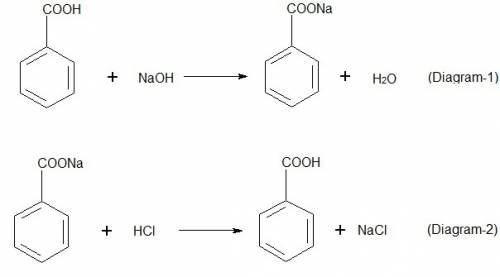
Explain the results for the tube in which 1.0 m naoh was added to benzoic acid. write an equation fo...
Questions


Mathematics, 09.03.2021 02:10

Health, 09.03.2021 02:10

Computers and Technology, 09.03.2021 02:10


Social Studies, 09.03.2021 02:10










Biology, 09.03.2021 02:10



Mathematics, 09.03.2021 02:10

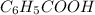 ), sodium benzoate and water (
), sodium benzoate and water (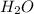 ) are formed because benzoic acid is weak acid and NaOH is strong base. The reaction is shown in diagram 1. To the tube of sodium benzoate, if 6 m HCl is added, sodium benzoate salt will react with HCl and produce benzoic acid and sodium chloride, NaCl. The reaction is shown in diagram 2.
) are formed because benzoic acid is weak acid and NaOH is strong base. The reaction is shown in diagram 1. To the tube of sodium benzoate, if 6 m HCl is added, sodium benzoate salt will react with HCl and produce benzoic acid and sodium chloride, NaCl. The reaction is shown in diagram 2.