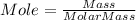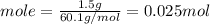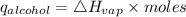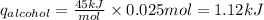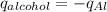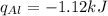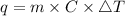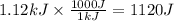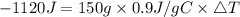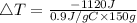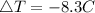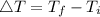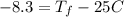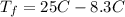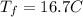Chemistry, 11.07.2019 22:30 reesewaggoner8
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the rubbing alcohol evaporates at 25 c, what is the final temperature of the aluminum?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the...
Questions

Spanish, 30.11.2020 21:40




English, 30.11.2020 21:40

Social Studies, 30.11.2020 21:40


Mathematics, 30.11.2020 21:40

Mathematics, 30.11.2020 21:40




English, 30.11.2020 21:40


Mathematics, 30.11.2020 21:40



Arts, 30.11.2020 21:40