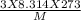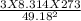Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
At 273 k and 1.00 x 10^-2 atm, the density of a gas is 1.24 x 10^-5 g/cm3. a. find vrms for the gas...
Questions


Chemistry, 08.07.2019 17:00

English, 08.07.2019 17:00


Mathematics, 08.07.2019 17:00

Biology, 08.07.2019 17:00

History, 08.07.2019 17:00

Mathematics, 08.07.2019 17:00

Mathematics, 08.07.2019 17:00

Mathematics, 08.07.2019 17:00



Mathematics, 08.07.2019 17:00

Mathematics, 08.07.2019 17:00

Computers and Technology, 08.07.2019 17:00


Social Studies, 08.07.2019 17:00

Spanish, 08.07.2019 17:00


Mathematics, 08.07.2019 17:00

 = √
= √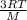 =√
=√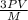 =√
=√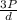 , where, R= Universal gas constant, T= Absolute temperature, P= Pressure, V= Volume of gas, d= Density of gas.
, where, R= Universal gas constant, T= Absolute temperature, P= Pressure, V= Volume of gas, d= Density of gas.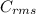 =√
=√