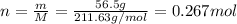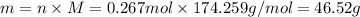Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

You know the right answer?
For each precipitation reaction, calculate how many grams of the first reactant are necessary to com...
Questions


Computers and Technology, 16.10.2021 18:00

Mathematics, 16.10.2021 18:00



Mathematics, 16.10.2021 18:00






Health, 16.10.2021 18:00

SAT, 16.10.2021 18:00

Business, 16.10.2021 18:00






Biology, 16.10.2021 18:00

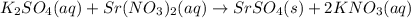
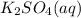 reacts with 1 mole of reactant 2
reacts with 1 mole of reactant 2 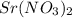 .
.