
Chemistry, 11.07.2019 19:30 calhountoiyonou0gjb
You are given a 1.50 g mixture of sodium nitrate and sodium chloride. you dissolve this mixture into 100. ml of water and then add an excess of 0.500 m silver nitrate solution. the white solid produced from the reaction is filtered, dried and measured. the white solid has a mass of 0.641 g. calculate the percent, by mass, of sodium chloride in the original unknown mixture.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
You are given a 1.50 g mixture of sodium nitrate and sodium chloride. you dissolve this mixture into...
Questions



English, 24.11.2020 21:10



Chemistry, 24.11.2020 21:10









Computers and Technology, 24.11.2020 21:10

Mathematics, 24.11.2020 21:10


SAT, 24.11.2020 21:10

Chemistry, 24.11.2020 21:10

Biology, 24.11.2020 21:10

 = 4.47 X
= 4.47 X 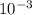 mole of solid AgCl is produced. This indicates 4.47 X
mole of solid AgCl is produced. This indicates 4.47 X 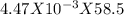 g of Nacl reacts to give 0.641 g of AgCl. So, mass percentage of NaCl present in mixture=
g of Nacl reacts to give 0.641 g of AgCl. So, mass percentage of NaCl present in mixture=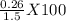 = 17.33 %.
= 17.33 %.

