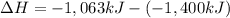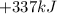Chemistry, 11.07.2019 19:30 Sadiecoyle37
What is the change in enthalpy for the single replacement reaction between solid zinc and cs2so4 in solution to produce cesium and znso4? zn + cs2so4 → 2cs + znso4 given: `"cs"_2"so"_4(aq): deltah_f °= –1,400 "kj"` `"znso"_4(aq): deltah_f °= –1,063 "kj"`

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1
You know the right answer?
What is the change in enthalpy for the single replacement reaction between solid zinc and cs2so4 in...
Questions

History, 27.01.2020 21:31

Social Studies, 27.01.2020 21:31








Mathematics, 27.01.2020 21:31


English, 27.01.2020 21:31





Geography, 27.01.2020 21:31

Health, 27.01.2020 21:31


Biology, 27.01.2020 21:31

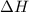 .
.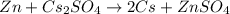
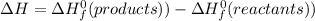
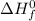 (products) and
(products) and